Cooltone
Home » Aesthetic Dermatology » Cooltone
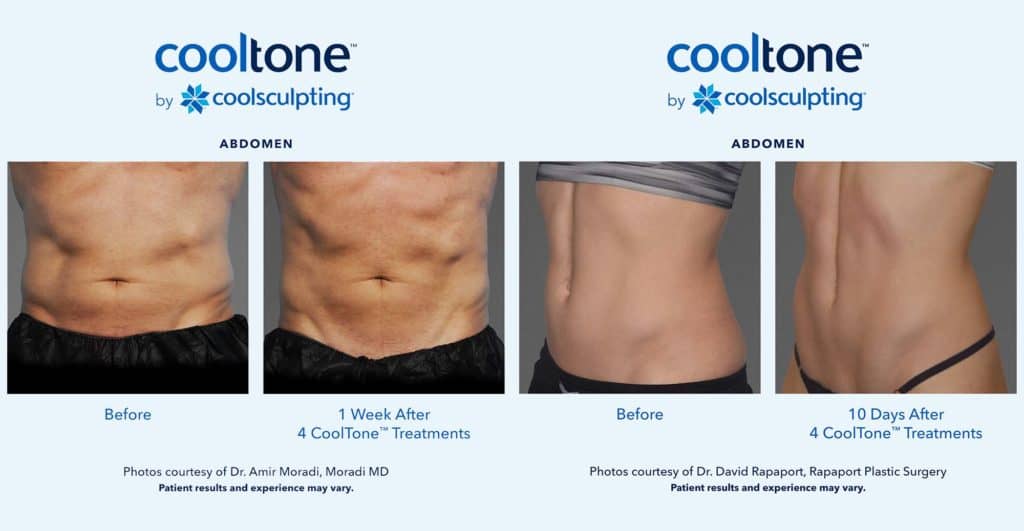
Why CoolTone?
CoolTone is an FDA cleared device clinically proven to strengthen, tone and firm the muscles it’s applied to. Areas that the device is indicated for include the abdomen, thighs, and buttocks. This treatment is completely non-invasive and can be used separately or in conjunction with CoolSculpting.
The CoolTone device is indicated for improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone is also indicated for strengthening, toning, and firming of the buttocks and thighs.
